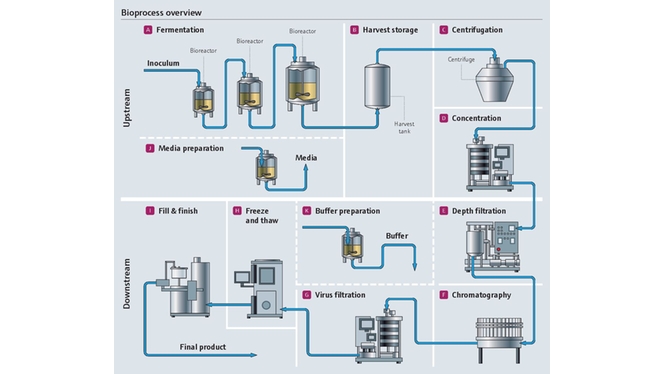
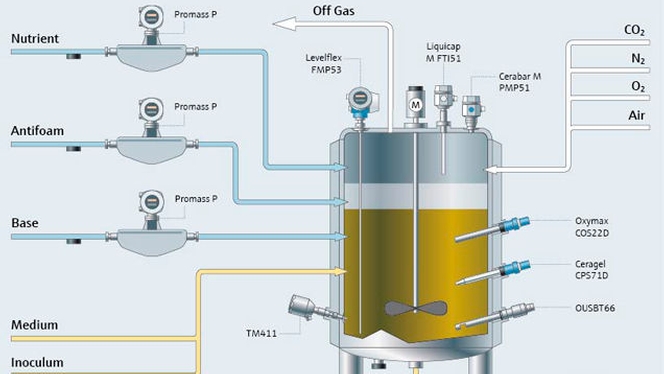
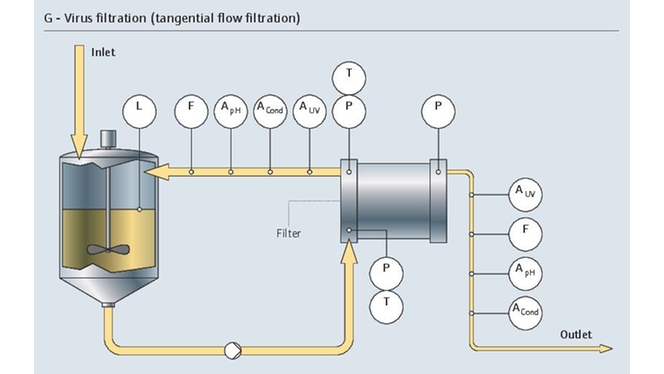
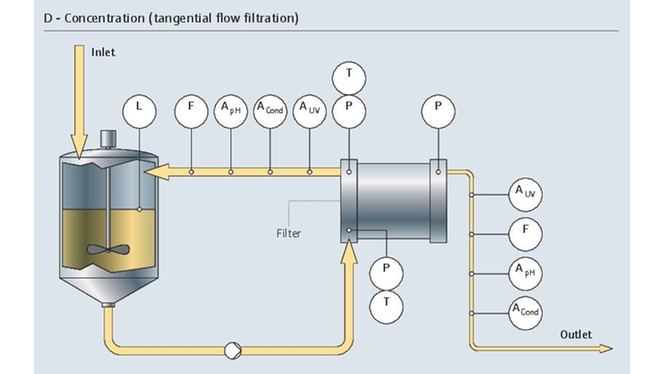
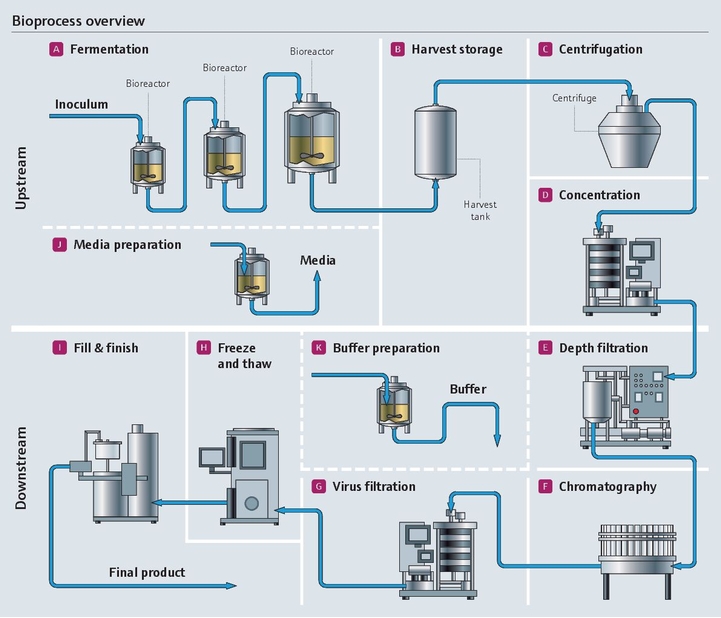
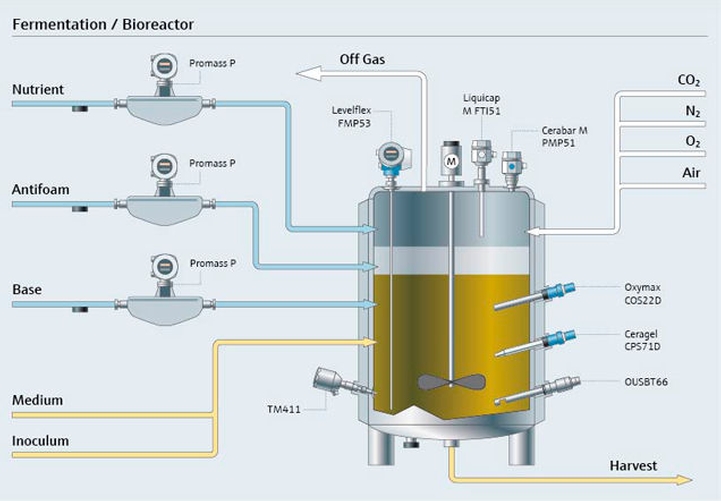
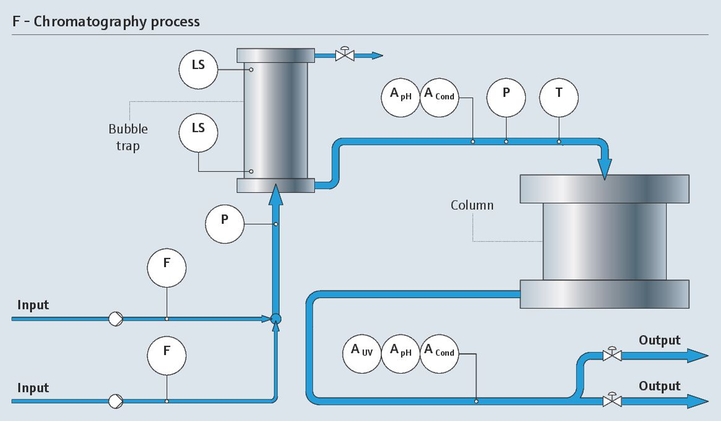
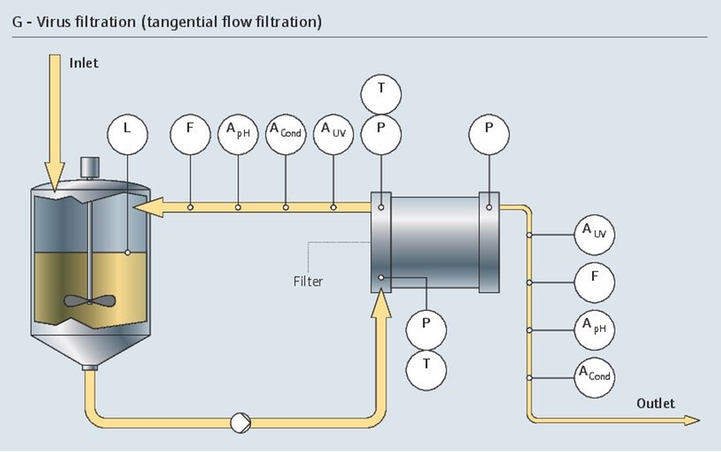
Project management in the pharma industry presents many challenges, few as complex as moving clinical innovations to manufacturing scale. Multiple industrial measurement instruments are installed and qualified during plant construction. Once operational, instruments must be maintained, verified and calibrated regularly. Our experience and expertise in project planning and engineering facilitate plant setup with early standardization, accelerating time to market and optimizing ongoing costs.
Key facts
3 weeks
faster time-to-market
thanks to perfectly engineered instrumentation packages
Learn how to standardize your projects

Expert partnering
In any pharmaceutical project, criticality and complexity grow with scale and the degree of process integration. Selecting and calibrating the right instrument for each application can be challenging, and yet is vital to ensuring measuring tasks are carried out optimally.
Our expertise in the field
Engaging our highly skilled and experienced engineers early in the project can reduce complexity and criticality in construction, as well as enhance ongoing plant performance.
- Select the right instrument for each application
- Reduce calibration downtime and assure permanent compliance with calibration engineering
- Boost overall performance with fieldbus engineering

Optimized planning
Standardization of instruments is key to reducing the complexity of large-scale pharmaceutical projects.
Our expertise in the field
An embedded Endress+Hauser project manager can help achieve a harmonized installed base, ensuring that all suppliers adhere to a standard instrument catalogue no matter where they are located.
- Collaborate closely from project outset to integrate standardization throughout
- Source standard instruments of Endress+Hauser global quality worldwide

Seamless implementation
Once the planning phase is completed, plans must be validated and implemented for timely and cost-effective execution.
Our expertise in the field
Our project managers can accelerate the start-up and help ensure ongoing operations are efficient and effective.
- Support factory acceptance testing (FAT), final validation and commissioning
- Deploy clearly defined and documented calibration, maintenance and compliance concepts
Benefits
Plan, manage and optimize your processes together with our experts. Not only are they able to offer you the complete range of instrumentation, they also have profound industry know-how to help you decrease the complexity of your pharmaceutical projects. Our engineers always strive to find the best match for your business needs.
Key facts
80%
fewer temperature measurement types through standardization
Key facts
3%
of initial investment saved on annual operating costs through standardization
Key facts
100%
matching instruments to user requirements - perfectly engineered, right the first time
How we can help
Endress+Hauser partners with customers from engineering through commissioning all the way to operational optimization, not only enabling plant planning and development projects to hit time and budget targets but also minimizing ongoing operating costs and plant downtime.
- Embedded Endress+Hauser engineers for standardization of best-fit instruments
- Integrated fieldbus and calibration engineering for maximized plant availability
- Optimized supply chain management, harmonized certification and documentation processes for minimized operating costs